Amines
NCERT Textbook Solution (Laptop/Desktop is best to view this page)
NCERT Solutions for Class 12 Chemistry Part 1 Chapter 13
Amines Class 12
Chapter 13 Amines Exercise Solutions
In text : Solutions of Questions on Page Number : 384
Q1 :
(i) Write structures of different isomeric amines corresponding to the molecular formula,
C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
Answer :
(i), (ii) The structures and their IUPAC names of different isomeric amines corresponding to the molecular formula, C4H11N are given below:
(a) CH3-CH2-CH2-CH2-NH2 Butanamine (10)
(b) 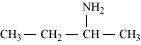
Butan-2-amine (10)
(c) 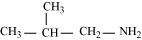
2-Methylpropanamine (10)
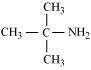 |
(d)
2-Methylpropan-2-amine (10)
(e) CH3-CH2-CH2-NH-CH3 N-Methylpropanamine (20)
(f) CH3-CH2-NH-CH2-CH3 N-Ethylethanamine (20) (g)
![]()
N-Methylpropan-2-amine (20)
(h)
N,N-Dimethylethanamine (3°)
(iii) The pairs (a) and (b) and (e) and (g) exhibit position isomerism.
The pairs (a) and (c); (a) and (d); (b) and (c); (b) and (d) exhibit chain isomerism. The pairs (e) and (f) and (f) and (g) exhibit metamerism.
All primary amines exhibit functional isomerism with secondary and tertiary amines and vice- versa.
Q2 :
Classify the following amines as primary, secondary or tertiary: (i)
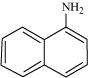
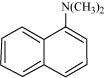 |
(ii)
(iii) (C2H5)2CHNH2
(iv) (C2H5)2NH
Answer :
Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii)
![]()
Q3 :
How will you convert?
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
(iii) Cl-(CH2)4-Cl into hexan-1, 6-diamine?
Answer :
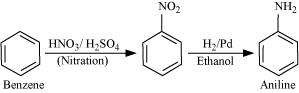 |
(i)
(ii)
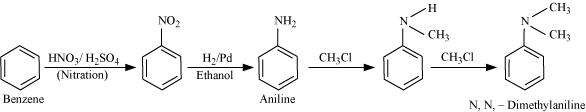
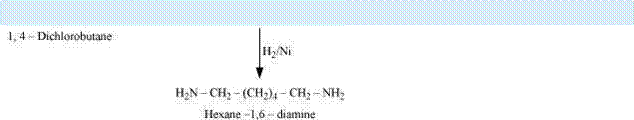 |
(iii)
Q4 :
Arrange the following in increasing order of their basic strength:
(i) C2H5NH2, C6H5NH2, NH3, C6H5CH2NH2 and (C2H5)2NH
(ii) C2H5NH2, (C2H5)2NH, (C2H5)3N, C6H5NH2
(iii)
CH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2.
![]()
Answer :
(i)
Considering the inductive effect of alkyl groups, NH3, C2H5NH2, and (C2H5)2NH can be arranged in the increasing order of their basic strengths as:
![]()
Again, C6H5NH2 has proton acceptability less than NH3. Thus, we have:
Due to the - I effect of C6H5 group, the electron density on the N-atom in C6H5CH2NH2 is lower than that on the N-atom in C2H5NH2, but more than that in NH3. Therefore, the given compounds can be arranged in the order of their basic strengths as:
![]()
(ii)
Considering the inductive effect and the steric hindrance of the alkyl groups, C2H5NH2, (C2 H5)2NH2, and their basic strengths as follows:
Again, due to the - R effect of C6H5 group, the electron density on the N atom in C6H5 NH2 is lower than that on the N atom in C2H5NH2. Therefore, the basicity of C6H5NH2 is lower than that of C2H5NH2. Hence, the given compounds can be arranged in the increasing order of their basic strengths as follows:
![]()
(iii)
Considering the inductive effect and the steric hindrance of alkyl groups, CH3NH2, (CH3)2NH, and (CH3)3N can be arranged in the increasing order of their basic strengths as:
In C6H5NH2, N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N - atom is delocalized over the benzene ring. In C6H5CH2NH2, N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N atom are more easily available for protonation in C6H5CH2NH2than in C6H5NH2 i.e., C6H5CH2 NH2 is more basic than C6H5NH2.
Again, due to the - I effect of C6H5 group, the electron density on the N - atom in C6H5CH2NH2 is lower than that on the N - atom in (CH3)3N. Therefore, (CH3)3N is more basic than C6H5CH2NH2. Thus, the given compounds can be arranged in the increasing order of their basic strengths as follows.
![]()
Q5 :
Complete the following acid-base reactions and name the products:
(i) CH3CH2CH2NH2 + HCl ![]()
(ii)
(C2H5)3N + HCl 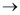
Answer :
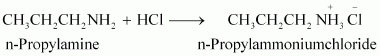 (i)
(i)
(ii) (C2H5)3N Triethylamine+ HCl → (C2H5)3N+HCl-Triethylammoniumchloride
Q6 :
Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Answer :
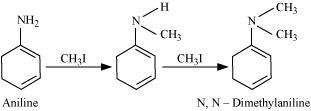 |
Aniline reacts with methyl iodide to produce N, N-dimethylaniline.
With excess methyl iodide, in the presence of Na2CO3 solution, N, N-dimethylaniline produces N, N, N-trimethylanilinium carbonate.
![]()
Q7 :
Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer :
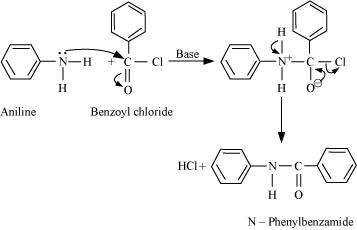
![]()
Q8 :
Write structures of different isomers corresponding to the molecular formula, C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Answer :
The structures of different isomers corresponding to the molecular formula, C3H9N are given below:
(a) 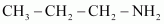
Propan-1-amine (10)
(b)
Propan-2-amine (10)
(c)
(d)
![]()
N,N-Dimethylmethanamine (30)
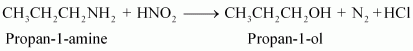 |
10amines, (a) propan-1-amine, and (b) Propan-2-amine will liberate nitrogen gas on treatment with nitrous acid.

Q9 :
Convert
(i) 3-Methylaniline into 3-nitrotoluene.
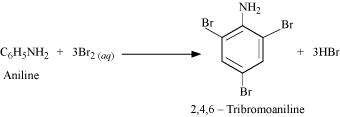
(ii) Aniline into 1,3,5-tribromobenzene.
Answer : (i)
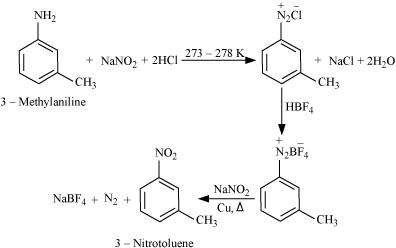
(ii)
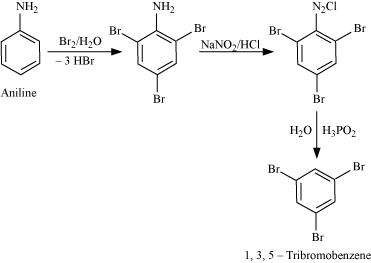
![]()
Exercise : Solutions of Questions on Page Number : 400
Q1 :
Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) (CH3)2 CHNH2 (ii) CH3(CH2)2NH2
(iii) CH3NHCH(CH3)2 (iv) (CH3)3CNH2
(v) C6H5NHCH3 (vi) (CH3CH2)2NCH3
(vii) m-BrC6H4NH2
Answer :
(i) 1-Methylethanamine (10 amine)
(ii) Propan-1-amine (10 amine)
(iii) N-Methyl-2-methylethanamine (20 amine)
(iv) 2-Methylpropan-2-amine (10 amine)
(v) N-Methylbenzamine or N-methylaniline (20 amine)
(vi) N-Ethyl-N-methylethanamine (30 amine)
(vii)
3-Bromobenzenamine or 3-bromoaniline (10 amine)
Q2 :
Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and N-methylaniline.
Answer :
(i) Methylamine and dimethylamine can be distinguished by the carbylamine test.
Carbylamine test: Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form foul-smelling isocyanides or carbylamines. Methylamine (being an aliphatic primary amine) gives a positive carbylamine test, but dimethylamine does not.
(ii) Secondary and tertiary amines can be distinguished by allowing them to react with Hinsberg's reagent (benzenesulphonyl chloride, C6H5SO2Cl).
Secondary amines react with Hinsberg's reagent to form a product that is insoluble in an alkali. For example, N, N - diethylamine reacts with Hinsberg's reagent to form N, N - diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg's reagent.
(iii) Ethylamine and aniline can be distinguished using the azo-dye test. A dye is obtained when aromatic amines react with HNO2 (NaNO2 + dil.HCl) at 0-5°C, followed by a reaction with the alkaline solution of 2-naphthol. The dye is usually yellow, red, or orange in colour. Aliphatic amines give a brisk effervescence due (to the evolution of N2 gas) under similar conditions.
​
(iv) Aniline and benzylamine can be distinguished by their reactions with the help of nitrous acid, which is prepared in situ from a mineral acid and sodium nitrite. Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
On the other hand, aniline reacts with HNO2 at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
(v) Aniline and N-methylaniline can be distinguished using the Carbylamine test. Primary amines, on heating with chloroform and ethanolic potassium hydroxide, form foul-smelling
isocyanides or carbylamines. Aniline, being an aromatic primary amine, gives positive carbylamine test. However, N-methylaniline, being a secondary amine does not.
Q3 :
Account for the following:
(i) pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is o, p- directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Answer :
(i)
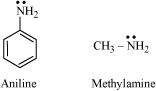 |
pKb of aniline is more than that of methylamine:
Aniline undergoes resonance and as a result, the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.
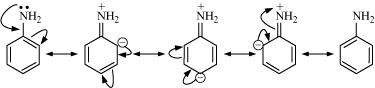
On the other hand, in case of methylamine (due to the +I effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine.
Thus, pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not:
Ethylamine when added to water forms intermolecular H - bonds with water. Hence, it is soluble in water.
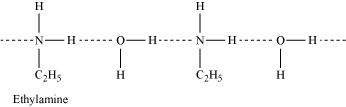
But aniline does not undergo H - bonding with water to a very large extent due to the presence of a large hydrophobic - C6H5 group. Hence, aniline is insoluble in water.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide:
![]()
Due to the +I effect of - CH3 group, methylamine is more basic than water. Therefore, in water, methylamine produces OH- ions by accepting H+ ions from water.
Ferric chloride (FeCl3) dissociates in water to form Fe3+ and Cl- ions.
![]()
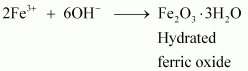 |
Then, OH- ion reacts with Fe3+ ion to form a precipitate of hydrated ferric oxide.
(iv) Although amino group is o,p - directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline:
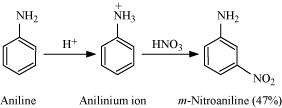 |
Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ion (which is meta-directing).
For this reason, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction:
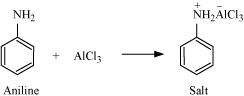 |
A Friedel-Crafts reaction is carried out in the presence of AlCl3. But AlCl3 is acidic in nature, while aniline is a strong base. Thus, aniline reacts with AlCl3 to form a salt (as shown in the following equation).
Due to the positive charge on the N-atom, electrophilic substitution in the benzene ring is deactivated. Hence, aniline does not undergo the Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines:
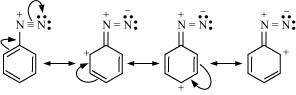 |
The diazonium ion undergoes resonance as shown below:
This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines:
Gabriel phthalimide synthesis results in the formation of 1° amine only. 2° or 3° amines are not formed in this synthesis. Thus, a pure 1° amine can be obtained. Therefore, Gabriel phthalimide synthesis is preferred for synthesizing primary amines.
Q4 :
Arrange the following:
(i) In decreasing order of the pKbvalues: C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
(b) C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
(iv) In decreasing order of basic strength in gas phase:
C2H5NH2, (C2H5)2NH, (C2H5)3N and NH3
(v) In increasing order of boiling point:
C2H5OH, (CH3)2NH, C2H5NH2
(vi) In increasing order of solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2.
Answer :
(i) In C2H5NH2, only one -C2H5 group is present while in (C2H5)2NH, two -C2H5 groups are present. Thus, the +I effect is more in (C2H5)2NH than in C2H5NH2. Therefore, the electron density over the N-atom is more in (C2H5)2NH than in C2H5NH2. Hence, (C2H5)2NH is more basic than C2H5NH2.
Also, both C6H5NHCH3 and C6H5NH2 are less basic than (C2H5)2NH and C2H5NH2 due to the delocalization of the lone pair in the former two. Further, among C6H5NHCH3 and C6H5NH2, the former will be more basic due to the +T effect of -CH3 group. Hence, the order of increasing basicity of the given compounds is as follows:
C6H5NH2 < C6H5NHCH3 < C2H5NH2 < (C2H5)2NH
We know that the higher the basic strength, the lower is the pKb values.
C6H5NH2 > C6H5NHCH3 > C2H5NH2 > (C2H5)2NH
(ii) C6H5N(CH3)2 is more basic than C6H5NH2 due to the presence of the +I effect of two -
CH3 groups in C6H5N(CH3)2. Further, CH3NH2 contains one -CH3 group while (C2H5)2NH contains two -C2H5 groups. Thus, (C2H5)2 NH is more basic than C2H5NH2.
Now, C6H5N(CH3)2 is less basic than CH3NH2 because of the-R effect of -C6H5 group. Hence, the increasing order of the basic strengths of the given compounds is as follows:
C6H5NH2 < C6H5N(CH3)2 < CH3NH2 < (C2H5)2NH
(iii)
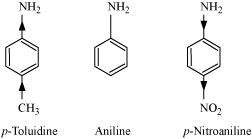 |
(a)
In p-toluidine, the presence of electron-donating -CH3 group increases the electron density on the N-atom.
Thus, p-toluidine is more basic than aniline.
On the other hand, the presence of electron-withdrawing
-NO2 group decreases the electron density over the N-atom in p-nitroaniline. Thus, p-nitroaniline is less basic than aniline.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
p-Nitroaniline < Aniline < p-Toluidine
(b) C6H5NHCH3 is more basic than C6H5NH2 due to the presence of electron-donating -CH3 group in C6H5NHCH3.
Again, in C6H5NHCH3, -C6H5 group is directly attached to the N-atom. However, it is not so in C6H5CH2NH2. Thus, in C6H5NHCH3, the -R effect of -C6H5 group decreases the electron density over the N-atom. Therefore, C6H5CH2NH2 is more basic than C6H5NHCH3.
Hence, the increasing order of the basic strengths of the given compounds is as follows: C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2.
(iv) In the gas phase, there is no solvation effect. As a result, the basic strength mainly depends upon the +I effect. The higher the +I effect, the stronger is the base. Also, the greater the number of alkyl groups, the higher is the +I effect. Therefore, the given compounds can be arranged in the decreasing order of their basic strengths in the gas phase as follows:
(C2H5)3N > (C2H5)2NH > C2H5NH2 > NH3
(v) The boiling points of compounds depend on the extent of H-bonding present in that compound. The more extensive the H-bonding in the compound, the higher is the boiling point. (CH3)2NH contains only one H-atom whereas C2H5NH2contains two H-atoms. Then,
C2H5NH2 undergoes more extensive H-bonding than (CH3)2NH. Hence, the boiling point of C2H5NH2 is higher than that of (CH3)2NH.
Further, O is more electronegative than N. Thus, C2H5OH forms stronger H-bonds than C2H5NH2. As a result, the boiling point of C2H5OH is higher than that of C2H5NH2 and (CH3)2NH.
Now, the given compounds can be arranged in the increasing order of their boiling points as follows:
(CH3)2NH < C2H5NH2 < C2H5OH
(vi) The more extensive the H-bonding, the higher is the solubility. C2H5NH2 contains two H- atoms whereas (C2H5)2NH contains only one H-atom. Thus, C2H5NH2 undergoes more extensive H-bonding than (C2H5)2NH. Hence, the solubility in water of C2H5NH2 is more than that of (C2H5)2NH.
Further, the solubility of amines decreases with increase in the molecular mass. This is because the molecular mass of amines increases with an increase in the size of the hydrophobic part. The molecular mass of C6H5NH2 is greater than that of C2H5NH2 and (C2H5)2NH.
Hence, the increasing order of their solubility in water is as follows: C6H5NH2 < (C2H5)2NH < C2H5NH2
![]()
Q5 :
How will you convert:
(i) Ethanoic acid into methanamine
(ii) Hexanenitrile into 1-aminopentane
(iii) Methanol to ethanoic acid
(iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine
(viii) Propanoic acid into ethanoic acid
Answer : (i)
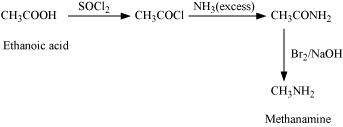
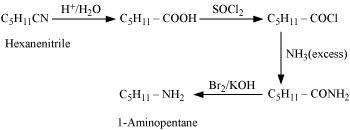 |
(ii)
(iii)
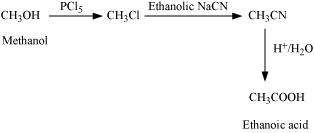
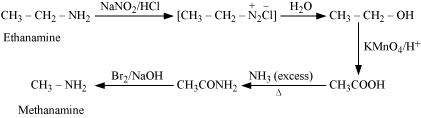 |
(iv)
(v)
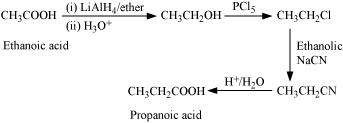
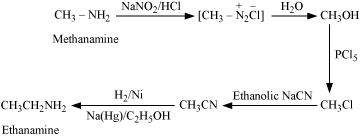 |
(vi)
(vii)
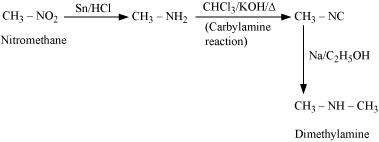
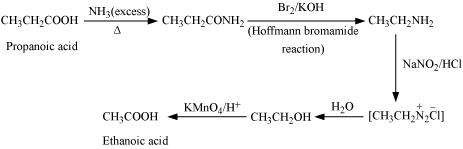 |
(viii)
![]()
Q6 :
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Answer :
Primary, secondary and tertiary amines can be identified and distinguished by Hinsberg's test. In this test, the amines are allowed to react with Hinsberg's reagent, benzenesulphonyl chloride
(C6H5SO2Cl). The three types of amines react differently with Hinsberg's reagent. Therefore, they can be easily identified using Hinsberg's reagent.
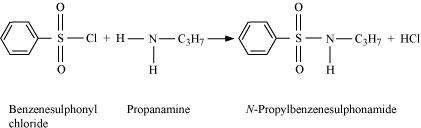 |
Primary amines react with benzenesulphonyl chloride to form N-alkylbenzenesulphonyl amide which is soluble in alkali.
Due to the presence of a strong electron-withdrawing sulphonyl group in the sulphonamide, the H-atom attached to nitrogen can be easily released as proton. So, it is acidic and dissolves in alkali.
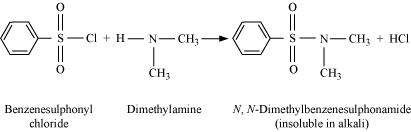 |
Secondary amines react with Hinsberg's reagent to give a sulphonamide which is insoluble in alkali.
There is no H-atom attached to the N-atom in the sulphonamide. Therefore, it is not acidic and insoluble in alkali.
On the other hand, tertiary amines do not react with Hinsberg's reagent at all.
Q7 :
Write short notes on the following:
(i) Carbylamine reaction (ii) Diazotisation
(iii) Hofmann's bromamide reaction (iv) Coupling reaction
(v) Ammonolysis (vi) Acetylation
(vii) Gabriel phthalimide synthesis.
Answer :
(i) Carbylamine reaction
Carbylamine reaction is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide,
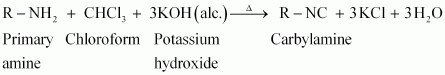 |
carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test.
For example,
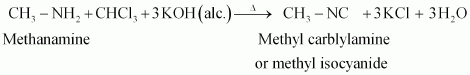
(ii) Diazotisation
Aromatic primary amines react with nitrous acid (prepared in situ from NaNO2and a mineral acid such as HCl) at low temperatures (273-278 K) to form diazonium salts. This conversion of aromatic primary amines into diazonium salts is known as diazotization.
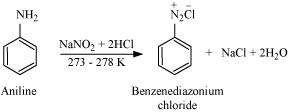 |
For example, on treatment with NaNO2and HCl at 273 - 278 K, aniline produces benzenediazonium chloride, with NaCl and H2O as by-products.
(iii) Hoffmann bromamide reaction
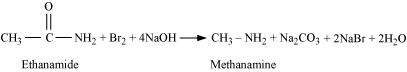
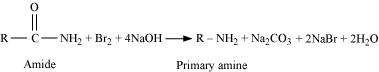 |
When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, a primary amine with one carbon atom less than the original amide is produced. This degradation reaction is known as Hoffmann bromamide reaction. This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon atom of the amide to the nitrogen atom.
For example,
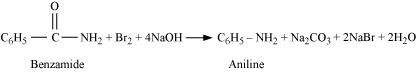 |
(iv) Coupling reaction
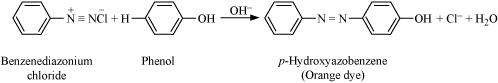 |
The reaction of joining two aromatic rings through the - N=N - bond is known as coupling reaction. Arenediazonium salts such as benzene diazonium salts react with phenol or aromatic amines to form coloured azo compounds.
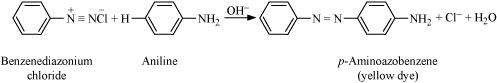
It can be observed that, the para-positions of phenol and aniline are coupled with the diazonium salt. This reaction proceeds through electrophilic substitution.
(v) Ammonolysis
When an alkyl or benzyl halide is allowed to react with an ethanolic solution of ammonia, it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (
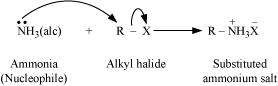 |
- NH2) group. This process of cleavage of the carbon-halogen bond is known as ammonolysis.
When this substituted ammonium salt is treated with a strong base such as sodium hydroxide, amine is obtained.
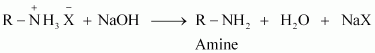
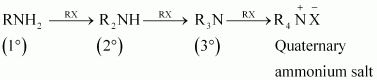 |
Though primary amine is produced as the major product, this process produces a mixture of primary, secondary and tertiary amines, and also a quaternary ammonium salt as shown.
(vi) Acetylation
Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule.
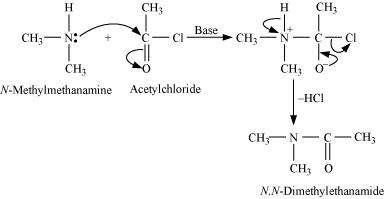 |
Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of - NH2or > NH group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right hand side, the HCl formed during the reaction is removed as soon as it is formed. This reaction is carried out in the presence of a base (such as pyridine) which is stronger than the amine.
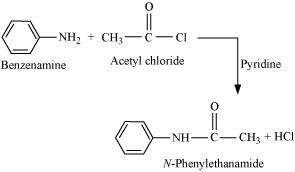
When amines react with benzoyl chloride, the reaction is also known as benzoylation. For example,
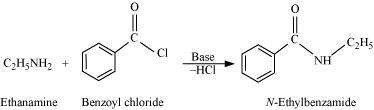
(vii) Gabriel phthalimide synthesis
Gabriel phthalimide synthesis is a very useful method for the preparation of aliphatic primary amines. It involves the treatment of phthalimide with ethanolic potassium hydroxide to form potassium salt of phthalimide. This salt is further heated with alkyl halide, followed by alkaline hydrolysis to yield the corresponding primary amine.
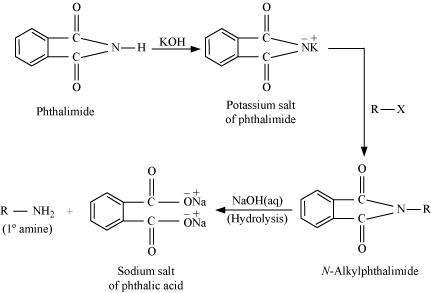
![]()
Q8 :
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2,4,6-tribromofluorobenzene
(v) Benzyl chloride to 2-phenylethanamine
(vi) Chlorobenzene to p-chloroaniline
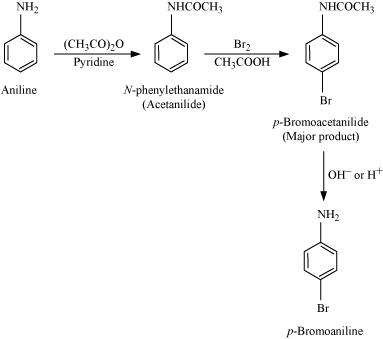
(vii) Aniline to p-bromoaniline
(viii) Benzamide to toluene
(ix) Aniline to benzyl alcohol.
Answer : (i)
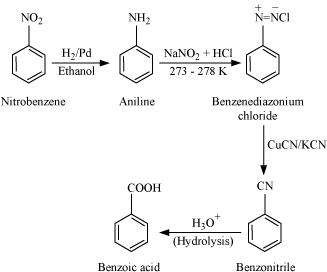
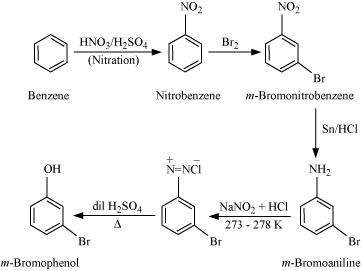 |
(ii)
(iii)
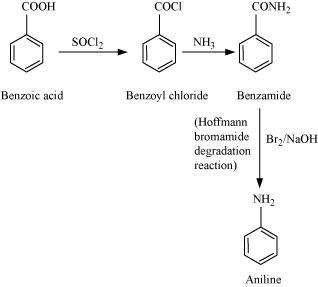
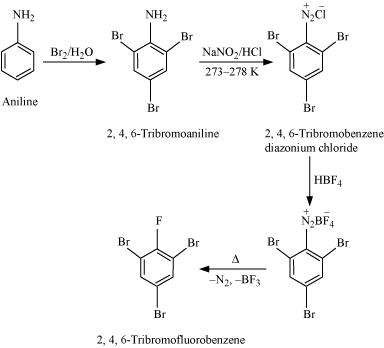 |
(iv)
(v)
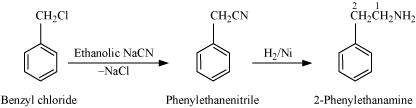
(vi)
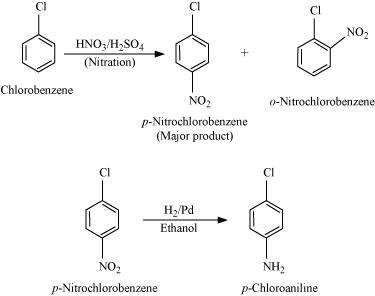
 |
(vii)
(viii)
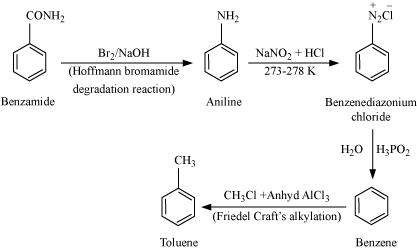
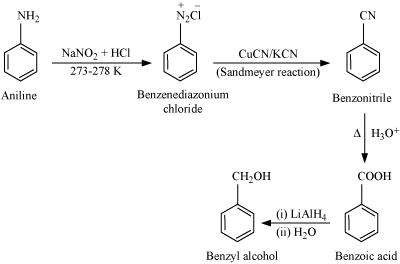 |
(ix)
![]()
Q9 :
Give the structures of A, B
and C in the following reactions: (i) 
(ii) ![]()
(iii) 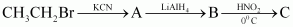
(iv) ![]()
(v) 
![]() (vi)
(vi)
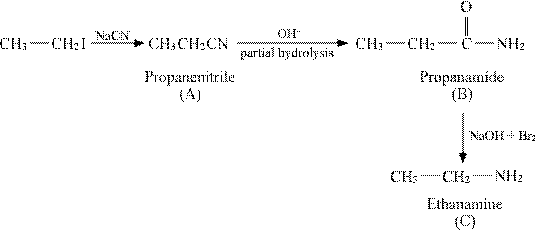 Answer : (i)
Answer : (i)
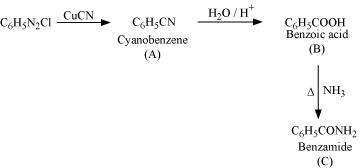 |
(ii)
(iii)
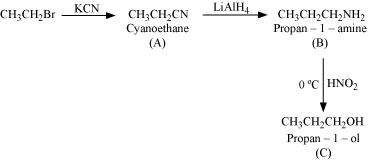
(iv)
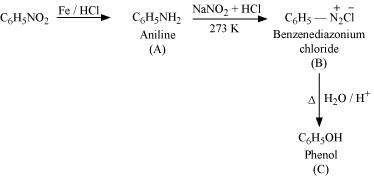
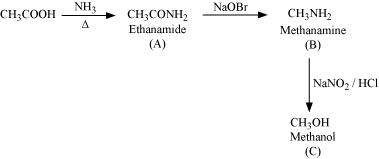 |
(v)
(vi)
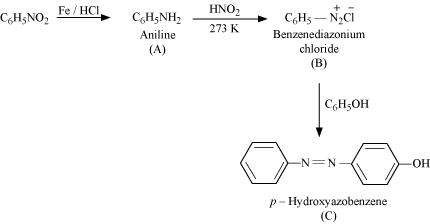
![]()
Q10 :
An aromatic compound 'A' on treatment with aqueous ammonia and heating forms compound 'B' which on heating with Br2 and KOH forms a compound 'C'
of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Answer :
It is given that compound 'C' having the molecular formula, C6H7N is formed by heating compound 'B' with Br2 and KOH. This is a Hoffmann bromamide degradation reaction. Therefore, compound 'B' is an amide and compound 'C' is an amine. The only amine having the molecular formula, C6H7N is aniline, (C6H5NH2).
Therefore, compound 'B' (from which 'C' is formed) must be benzamide, (C6H5CONH2).
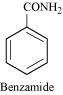
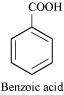 |
Further, benzamide is formed by heating compound 'A' with aqueous ammonia. Therefore, compound 'A' must be benzoic acid.
The given reactions can be explained with the help of the following equations:
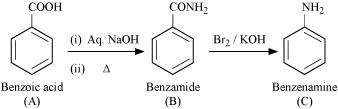
![]()
Q11 :
Complete the following
reactions: (i) 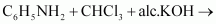
(ii) ![]()
(iii) 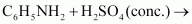
(iv) ![]()
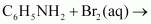 (v)
(v)
(vi) ![]()
 (vii)
(vii)
Answer : (i)

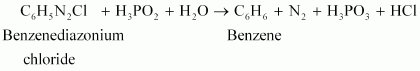 |
(ii)
(iii)
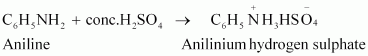
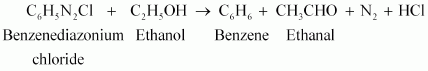 |
(iv)
(v)
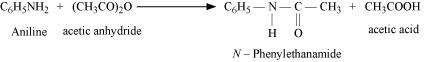 |
(vi)
(vii)
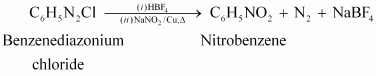
![]()
Q12 :
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Answer :
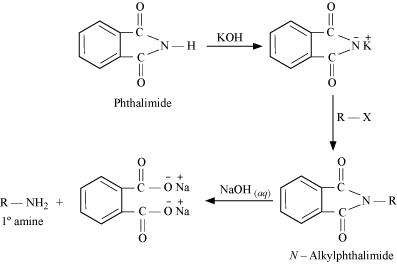 |
Gabriel phthalimide synthesis is used for the preparation of aliphatic primary amines. It involves nucleophilic substitution (SN2) of alkyl halides by the anion formed by the phthalimide.
But aryl halides do not undergo nucleophilic substitution with the anion formed by the phthalimide.
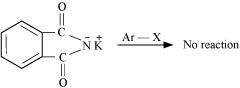
Hence, aromatic primary amines cannot be prepared by this process.
Q13 :
Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid. Answer :
(i)
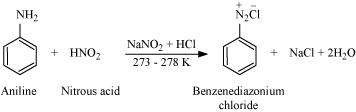 |
Aromatic amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 - 278 K to form stable aromatic diazonium salts i.e., NaCl and H2O.
(ii) Aliphatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) to form unstable aliphatic diazonium salts, which further produce alcohol and HCl with the evolution of N2 gas.
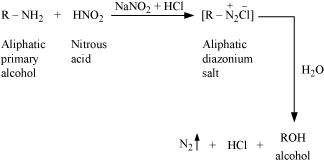
Q14 :
Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid.
Answer :
(i)
 |
Aromatic amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 - 278 K to form stable aromatic diazonium salts i.e., NaCl and H2O.
(ii) Aliphatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) to form unstable aliphatic diazonium salts, which further produce alcohol and HCl with the evolution of N2 gas.
